In the last part of the tutorial, we are going to go through some ways in which you can investigate the impact of DNA methylation. The examples that we outline here are just some of the examples that you can use as figures in your paper.
Firstly, we need to prepare our files to only include the first 3 columnes (chromosome, chromosome start and chromosome end) in the bedGraph file (i.e. creating a bed3 file).
This is required for the cpgtools.
We need to first remove the "track" line (i.e. the first line) and exract columns 1, 2 and 3.
$ grep -v "track" SRR534177_colWT_trimmed_bismark_bt2.deduplicated.sorted_CpG.bedGraph | \
cut -f1-3 > SRR534177_colWT_trimmed_bismark_bt2.deduplicated.sorted_CpG.bed
Lets create a loop on the command-line to do this on all files:
$ for i in *.bedGraph; do
grep -v "track" $i | cut -f1-3 > `basename $i .bedGraph`.bed
done
As an example, we will only be running CpG bed files, so try all the other contexts as well
With CpG sites found in both samples, we now want to see what genes that find what genes that these sites overlap with.
To do this, we're going to use the TAIR10 gene bedfile (converted from GFF3 annotation file) and the bedtools suit of tools.
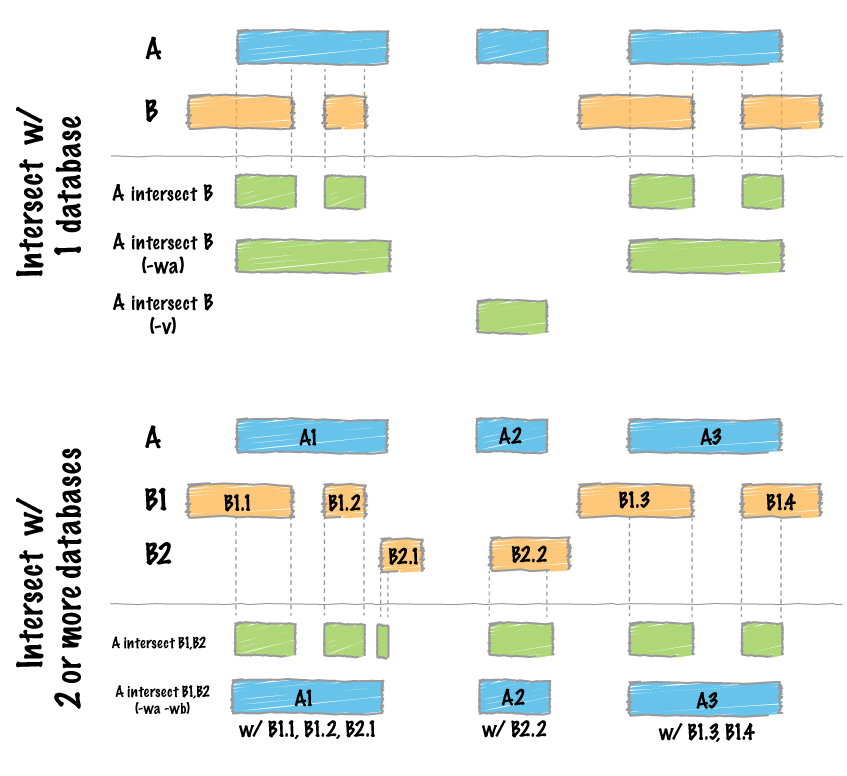
To intersect the CpG sites with gene intervals, we'll use bedtools intersect
Lets intersect our bed file with the TAIR10 genes and extract the CpG columns from the first file (chromosome, chromosome start and chromosome end) and the gene name from the second file (7th column of the output)
$ bedtools intersect -a SRR534177_colWT_trimmed_bismark_bt2.deduplicated.sorted_CpG.bed \
-b TAIR10_genes.bed -wo | cut -f1,2,3,7
$ bedtools intersect -a SRR534239_met1_trimmed_bismark_bt2.deduplicated.sorted_CpG.bed \
-b TAIR10_genes.bed -wo | cut -f1,2,3,7
You can actually extract this information with cpgtools using the CpG_to_gene.py command.
This command takes a bed file (3 column) input and gene-associated bed file to determine sites which overlap.
$ CpG_to_gene.py -i SRR534239_met1_trimmed_bismark_bt2.deduplicated.sorted_CpG.bed \
-r TAIR10_genes.bed -o output
$ head -n 20 output.associated_genes.txt
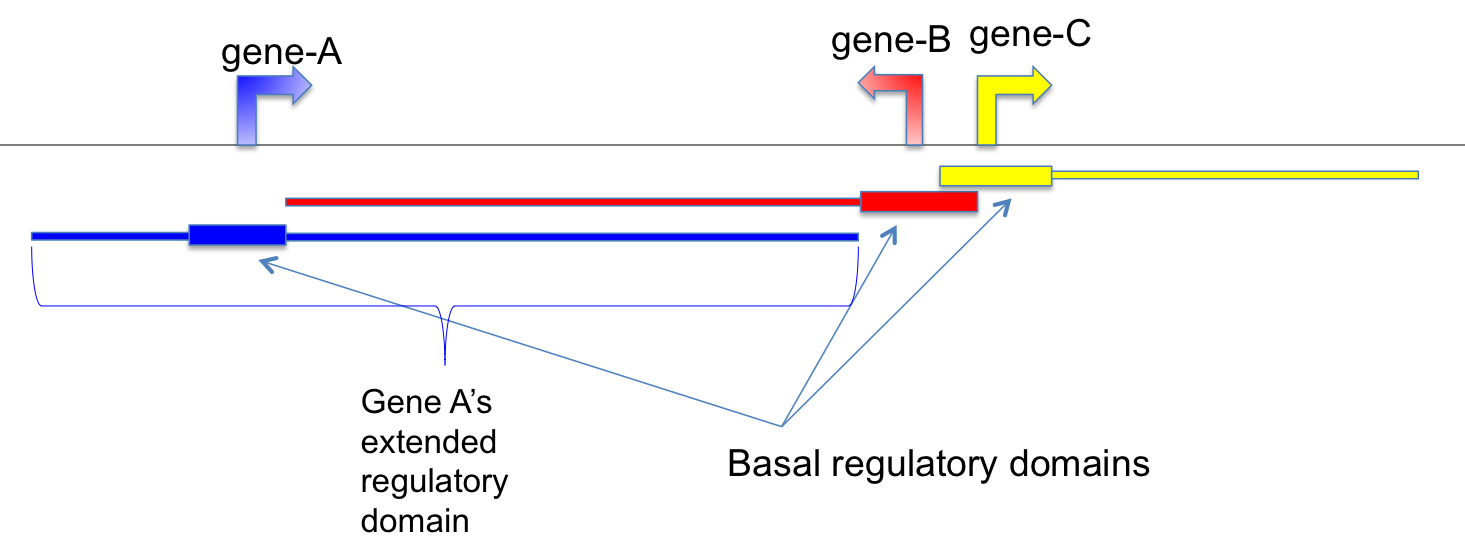
#The last column contains genes whose extended regulatory domain are overlapped with the CpG
#The 2nd last column contains genes whose basal regulatory domain are overlapped with the CpG
#"//" indicates no genes are found
1 4107 4108 AT1G01010 //
1 5458 5459 // AT1G01020
1 8642 8643 AT1G01020 //
1 8658 8659 AT1G01020 //
1 8661 8662 AT1G01020 //
1 12227 12228 AT1G01020 //
1 12302 12303 AT1G01020 //
1 12394 12395 AT1G01020 //
1 12726 12727 AT1G01030;AT1G01020 //
1 16759 16760 AT1G01030 //
1 19212 19213 AT1G01040 //
1 23296 23297 AT1G01040 //
1 23844 23845 AT1G01040;AT1G01046 //
1 24187 24188 AT1G01046 //
1 24189 24190 AT1G01046 //
1 24195 24196 AT1G01046 //
1 24235 24236 AT1G01046 //
cpgtools define these regulatory domains as:
"Basal regulatory domain is a user-defined genomic region around the TSS (transcription start site). By default, from TSS upstream 5 Kb to TSS downstream 1 Kb is considered as the gene’s basal regulatory domain. When defining a gene’s basal regulatory domain, the other nearby genes are ignored (which means different genes’ basal regulatory domain can be overlapped.)
Extended regulatory domain is a genomic region that is further extended from basal regulatory domain in both directions to the nearest gene’s basal regulatory domain but no more than the maximum extension (specified by ‘-e’, default - 1000 kb) in one direction. In other words, the “extension” stops when it reaches other genes’ “basal regulatory domain” or the extension limit, whichever comes first."
Lets now have a look at how methylation sites are distributed over the two chromosomes we analysed, chromosome 1 and the chloroplast.
These plots are a great way to compare two regions.
This cpgtools command takes the CpG bed file, sample name and the total sequence length of each chromosome.
$ CpG_distrb_chrom.py -i SRR534177_colWT_trimmed_bismark_bt2.deduplicated.sorted_CpG.bed \
-n colWT -s TAIR.chrom.sizes -o chromDist
This produces a number of figures, each that displaye slightly different metrics:
$ ls -l chromDist*
chromDist.CpG_perMb.pdf
chromDist.CpG_percent.pdf
chromDist.CpG_total.pdf
Perhaps one of the most common plots seen in DNA methylation studies is the centred gene methylation plot, where the density of methylation sites are compared to the body of the gene and upstream/downstream regions.
Again, here we use a cpgtools command takes the gene coordinates and CpG bed file to produce the density plot.
CpG_density_gene_centered.py -r TAIR10_genes.bed \
-i SRR534177_colWT_trimmed_bismark_bt2.deduplicated.sorted_CpG.bed \
-o CpG_density
Be aware, this is density of sites (count) rather than methylation level.